FDA Finalizes the “Healthy” Claim
On December 27, 2024, FDA published a final rule to update the definition of the “healthy” implied nutrient content claim (see 89 Fed. Reg. 106064). Since 1994, FDA has recognized that using the term “healthy” on a product implies a certain level of nutrients that may help consumers maintain healthy dietary practices. The previous definition of "healthy" under 21 CFR 101.65 was primarily based on the levels of individual nutrients in foods, such as fat, sugar, and sodium. This approach led to some nutrient-dense foods, like salmon, being excluded because they contained higher levels of certain nutrients (e.g., fat), even though they are beneficial to health. Conversely, some foods high in added sugars could still be labeled as "healthy" if they met the specific nutrient criteria.
The new rule shifts the focus to food groups and dietary patterns, aligning with the latest nutrition science and dietary guidelines. It emphasizes the overall nutritional quality of foods and their role in a healthy diet. This rule is part of a broader FDA strategy to address diet-related diseases in line with the White House initiative on hunger, nutrition, and health. FDA’s efforts to improve nutrition also include the voluntary sodium reduction goals and the pending front-of-package nutrition labeling rule.
Key Changes in the New Rule
The major changes introduced by the final rule are as follows:
- In order to use the term “healthy,” or similar terms (e.g., “health,” “healthful,” “healthier,” etc.) on the label or labeling of a packaged food product, that food product must meet a set of parameters (further details are below).
- The parameters established by the rule are based on a framework that sorts food products into (1) food groups by category and (2) evaluates those products based on nutrients to limit (NTL) for the “healthy” claim (i.e., salt, added sugar, fat).
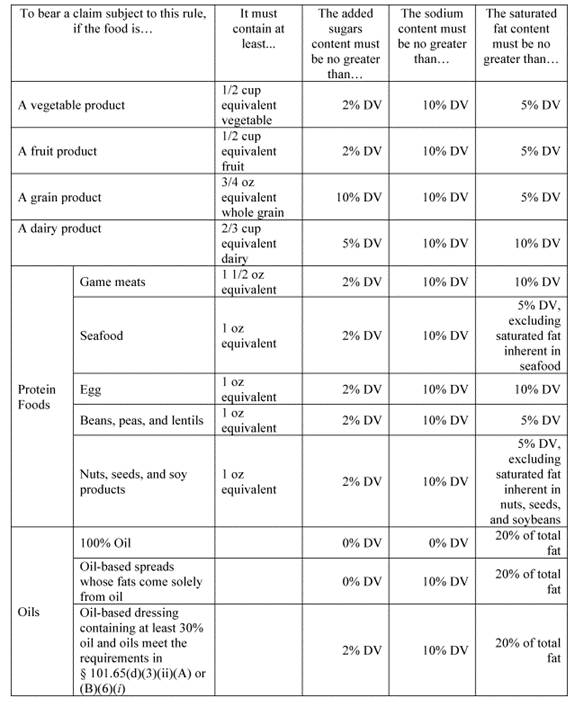
- Establishes that “food groups” for the purposes of the “healthy” claim refers to the groups established in the Dietary Guidelines, 2020-2025 and includes: vegetables, fruits, dairy, grains, protein foods, and oils.
Parameters
More specifically, to make a “healthy” claim, a food item must now meet specific conditions based on the Reference Amount Customarily Consumed (RACC) and limitations on the Daily Value (DV) percentages of added sugar, sodium, and fat. “Food groups” for purposes of the “healthy” claim are from the food groups recommended in the Dietary Guidelines, 2020-2025, which establish food group equivalents (FGEs) that identify qualifying amounts of food from each food group. For example, there must be at least ½ cup equivalent of a vegetable in a vegetable food product to qualify for the health claim. The second requirement is that individual food products that fall into one of the FGEs established above, and mixed products, main dishes, and meals, must then also meet specific limits for added sugars, saturated fat, and sodium based on a percentage of the DV for these nutrients. For example, a vegetable product must contain at least ½ cup equivalent of vegetables and no more than 2% of the DV for added sugar, 10% of the DV for sodium, and 5% of the DV for saturated fat.
Mixed products must contain at least one total FGE with no less than 1/4 FGE from at least two food groups, the added sugar content can be no greater than 10% of the DV, the sodium value can be no greater than 15% of the DV, and the saturated fat content can be no greater than 10% of the DV.
Main dishes, defined in 21 CFR 101.12(m) as a food that makes a major contribution to a meal by weighing at least 6 ounces per labeled serving, contains no less than 40 grams of food or combinations of foods, from at least two of the following four food groups:
1. Breads, cereal, rice, and pasta
2. Fruits and vegetables
3. Milk, yogurt, and cheese
4. Meat, poultry, fish, dry beans, eggs, and nuts
A main dish does not include sauces, gravies, condiments, relishes, pickles, olives, jams, jellies, syrups, breading, or garnishes and is in a form that is commonly understood to be a main dish (e.g., not a dessert or beverage.)
A main dish must have at least two total FGEs with no less than 1/2 FGE from at least two food groups, the added sugars can be no greater than 15% of the DV, the sodium content can be no greater than 20% of the DV, and the saturated fat can be no greater than 15% of the DV.
Meals, defined in 21 CFR 101.12(I) as a food that makes a major contribution to the total diet by weighing at least 10 ounces per labeled serving, and containing not less than three 40 grams portion of food, or combinations of foods, from two or more of the same food categories established above for main dishes. Meals must have three total FGEs with no less than 1/2 FGE from at least three food groups, the added sugars can be no greater than 20% of the DV, the sodium content can be no greater than 30% of the DV, and the saturated fat can be no greater than 20% of the DV.
Main Dishes and Meals have their own separate requirements regarding the FGEs and limits on added sugar, sodium, and saturated fat that are more flexible than the requirements for individual food items.
Certain foods automatically qualify for a healthy claim, including vegetables, fruits, whole grains, fat-free and low-fat dairy, lean meat, seafood, eggs, beans, peas, lentils, nuts, seeds, and water, tea, and coffee with less than 5 calories per RACC.
The exact parameters for each established food group are detailed in the following charts below.

Changes from the Proposed Rule to the Final Rule
The final rule also includes several significant changes from the 2022 proposed rule. The “healthy” criteria now apply to individual foods with a RACC of 50 grams or less or 3 tablespoons or less on a per 50-gram basis, allowing foods consumed in small amounts that are recommended for healthy dietary patterns to qualify for the claim. The exemption for raw, whole fruits and vegetables has been expanded. The Food Group Equivalents (FGE) criteria have been adjusted, such as reducing the FGE for dairy from three-quarter cup-equivalent to two-thirds cup-equivalent. There is also additional flexibility for combination foods in the proportions required for FGEs, and fruit and vegetable powders may now be considered in the calculation of FGEs for the healthy claim. The nutrient limits for sodium in mixed products have increased from 10% to 15%, and added sugars in whole grains from 5% to 10%. Additionally, inherent saturated fats in seafood are excluded from nutrient limits.
The rule also imposes recordkeeping requirements for foods bearing the “healthy” claim where the FGE contained in the product is not apparent from the label of the food. In such cases, the manufacturer must keep records for a period of at least two years after introducing the food into interstate commerce.
Foods that do not qualify for the “healthy” claim can still use other nutrient content claims, such as “low saturated fat,” and health claims that show how certain foods can reduce the risk of disease or health-related conditions.
Public Feedback
The FDA received approximately 400 comments on the proposed rule. Many comments reflected differing opinions on the rule, with some arguing that “healthy” is subjective and difficult to define. FDA responded that the definition is based on the most recent dietary guidelines and is designed to be broad and flexible, using percentages of the Daily Value rather than absolute values. FDA also plans to undertake consumer education efforts related to the healthy claim and is planning on releasing a “healthy” symbol that manufacturers can use on their product packaging.
Next Steps
For companies that sell products using the “healthy” claim or that might be impacted by this final rule, the next steps involve reviewing the updated definition and criteria for the “healthy” claim to understand how products will be affected. The compliance date for this final rule is February 25, 2028. Keller and Heckman can assist companies in reviewing product labeling to ensure compliance with the new “healthy” definition, including making necessary changes to labeling and marketing materials. Keller and Heckman will continue to monitor developments, including any additional guidance or updates from FDA regarding the implementation of the new rule and related initiatives.
We note that it is possible that the status and timeline related to this rulemaking could change. On January 20, 2025, President Donald Trump issued a Regulatory Freeze Executive Order to temporarily halt rulemaking and similar regulatory activity. The purpose of the freeze is to allow the new administration to review recently proposed and promulgated rules. After review, actions subject to the freeze will either move forward unchanged, be withdrawn, or be subject to additional rulemaking activity. Under the order, federal agencies must: (1) refrain from proposing or issuing any rules until the new administration reviews and approves the rule; (2) immediately withdraw any rules that have been sent to the Office of the Federal Register but not yet published so that they can be reviewed and approved; and (3) consider postponing the effective date for any rules that have not yet taken effect for 60 days for the purpose of reviewing any questions of fact, law, and policy that the rules may raise.
For example, the effective date of the final rule was originally set for February 25, 2025. The final rule will need to be reviewed and approved by a Trump-appointed FDA head before it can proceed, and the effective date of the rule could be postponed as a result to allow for this review. Depending on the review, the rule might also be modified or even withdrawn.
If you have any questions about FDA’s final rule for the “healthy” claim, please feel free to contact Partner Eve Pelonis (pelonis@khlaw.com), Associate Kaitlyn Johnson (johnsonk@khlaw.com), or your existing contact at Keller and Heckman LLP.