FDA Proposes Front-Of-Pack Nutrition Labeling Rule
On January 16, 2025, FDA published a proposed rule that would require a front-of-package (FOP) nutrition label on most packaged foods. See 90 Fed. Reg. 5426. The proposed FOP nutrition label would highlight the amount of saturated fat, sodium, and added sugars in a serving of food, as well as interpret the relative amounts of these nutrients, to help consumers quickly and easily identify how foods can be part of a healthy diet.
The proposed rule would add 21 CFR 101.6, requiring the inclusion of a Nutrition Info box on the principal display panel (PDP) of most foods that are required to display the Nutrition Facts label. The Nutrition Info box is intended to provide interpretive nutrition information in a convenient format that complements the Nutrition Facts Panel. The Nutrition Info box format is similar in style to the Nutrition Facts Panel and would be required to be placed on the upper third of the PDP. The box would be required to include:
- The title “Nutrition info;”
- A “Per serving” subheading and a statement of the serving size in household measure only;
- A percent daily value subheading above the declaration of the quantitative percent daily value and the interpretive “Low,” “Med,” and “High” descriptions; and
- Information on only saturated fat, sodium, and added sugars.
An example of the Nutrition Info box is as follows:
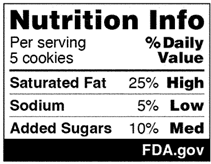
Due to the significance of saturated fat, sodium, and added sugars in building a healthy dietary pattern, FDA is proposing that only these three nutrients be included in the Nutrition Info box. This aligns with the Dietary Guidelines for Americans 2020-2025 recommendations and FDA’s recent final rule amending the “healthy” claim. Further, FDA’s research on FOP nutrition labeling, including focus groups and consumer surveys, demonstrated that consumers more easily understand simpler schemes that do not combine nutrients to limit and nutrients to get enough of. Ultimately, FDA decided that a scheme focusing only on nutrients to limit would be more valuable to help consumers build and maintain healthy dietary practices. FDA also chose not to include a calorie disclosure in the Nutrition Info box because it would not provide customers with new, interpretive information. However, voluntary calorie disclosures could accompany the Nutrition Info box in formats such as the following:
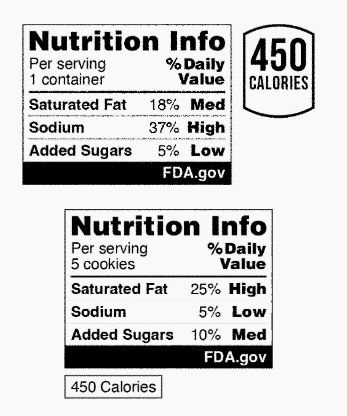
The interpretive descriptions of saturated fat, sodium, and added sugars are set at a proposed range of 5% daily value or less for “Low,” 6% to 19% daily value for “Med,” and 20% daily value or more for “High.” These ranges are based on longstanding consumer and nutrition education initiatives and existing regulatory definitions for nutrient content claims. FDA is requesting comments regarding data and information on alternative criteria for the interpretive descriptions that could support a goal of providing information for the levels, as well as use of the “Low” categorization for products that declare 0% for any of the three nutrients.
FDA is proposing ways to modify the Nutrition Info box for labels that use an aggregate or dual-column Nutrition Facts display and intermediate sized packages. Specifically, packaged foods that use an aggregate display must use a Nutrition Info box for each product contained in the package. Packages bearing a dual-column Nutrition Facts label presenting “per serving” and “per individual unit” information must only present the Nutrition Info box in the standard “per serving” format; however, packages that present both “as packaged” and “as prepared” nutrition information must present the Nutrition Info in the “as packaged” format. Foods in intermediate sized packages as defined in 21 CFR 101.9 may display a modified Nutrition Info box that displays only interpretive information. Examples of each of these modified Nutrition Info boxes are shown below:

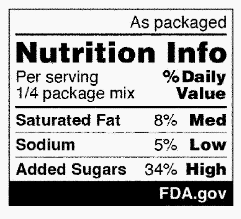
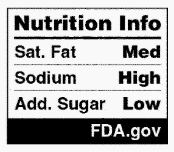
The proposed rule would apply to all foods covered under 21 CFR 101.9 marketed for people ages 4 and older unless exempted. The rule would not apply to foods marketed for children under 4 years old due to their specific nutritional needs; however, FDA is accepting comments on the nutritional needs of young children, as well as the need for or value of interpretive nutrition information on packages of food for young children to inform any future FOP policy. In addition, most dietary supplements would be exempt from the rule, but comments are invited on this exemption. Further, the proposed rule includes exemptions for foods exempt from nutrition labeling under 21 CFR 101.9(j).
Finally, while statements such as “low” and “high” would otherwise be considered nutrient content claims if voluntarily included on the PDP, FDA determined that the statements required in the Nutrition Info box will not constitute nutrient content claims because they are required to be displayed on all foods subject to the rule. However, any claim about the levels of the nutrients outside of the Nutrition Info box would still be considered a nutrient content claim that must comply with existing requirements under section 403(r) of the Food, Drug, and Cosmetic Act and the relevant nutrient content regulations, e.g., 21 CFR 101.13.
FDA is accepting comments on the proposed rule until May 16, 2025, at regulations.gov under docket number FDA-2024-N-2910. Keller and Heckman would be happy to prepare comments and will continue to monitor and relay updates on FDA’s proposed FOP labeling.
We note that the status and timeline related to the FOP nutrition label rulemaking is subject to change. On January 20, 2025, President Donald Trump issued a Regulatory Freeze Executive Order to temporarily halt rulemaking and similar regulatory activity. The purpose of the freeze is to allow the new administration to review recently proposed and promulgated rules. After review, actions subject to the freeze will either move forward unchanged, be withdrawn, or be subject to additional rulemaking activity. Under the order, federal agencies must: (1) refrain from proposing or issuing any rules until the new administration reviews and approves the rule; (2) immediately withdraw any rules that have been sent to the Office of the Federal Register but not yet published so that they can be reviewed and approved; and (3) consider postponing the effective date for any rules that have not yet taken effect for 60 days for the purpose of reviewing any questions of fact, law, and policy that the rules may raise.
If you have any questions about the Front-Of-Pack Nutrition Labeling Rule, please feel free to contact Partner Eve Pelonis (pelonis@khlaw.com), Associate Codi Walton (walton@khlaw.com), or your existing contact at Keller and Heckman LLP.